The Science of Snowmaking
This complete guide to the science of snowmaking is designed to give you a solid background in the fundamentals of making man-made snow. This will give you a solid understanding of how different types of snow guns work, and allow you to assess if your equipment is running properly and troubleshoot any problems that occur. Understanding the science first will help you avoid costly mistakes in the beginning of your snowmaking journey.
Introduction
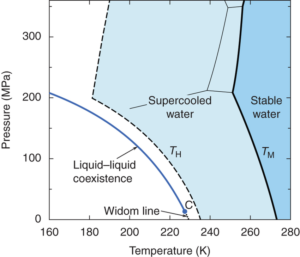
The snowmaking process turns water into small ice particles we call snow. In order to make snow, water must freeze to form these ice crystals in the air before reaching the ground. This sounds like a simple process – just spray water into the air when the temperature is below freezing, right? Contrary to what you may have learned, water does not always freeze at 32°F (0°C). In fact, water can exist in supercooled states at temperatures as low as -40°F (see image from this paper). This is because in order for the phase change to occur, a small nucleus or seed of ice must be formed inside the liquid. This process is called nucleation, and is critical to the snowmaking process.
Try spraying water into the air on a cold day, and you’ll realize that all that is produced is freezing rain! The water droplets will not freeze as they fall through the air, instead freezing on contact with the ground. In this case, the ground serves as a nucleation site for the droplets. If the droplets are to freeze in the air, nucleation sites must be provided. As you can begin to see, the snowmaking process is fairly complex, and nucleation is just one piece of the puzzle.
How does snowmaking work? In order to turn water into snow, several things need to occur:
- Produce water droplets that are the right size
- Cool the droplets to below the freezing point
- Provide nucleation sites for the droplets
- Allow the droplets to freeze before hitting the ground
We will go into each one of these in more detail, and you will see how the pieces fit together to make snow!
Droplet Size
In order to make small ice particles from water, the water must first be broken up into small droplets. When the water is broken up into droplets, the total surface area of the liquid is therefore increased, exposing more liquid to the ambient atmosphere and allowing for a greater cooling effect. The size of these droplets is important – too big and the droplets will not cool down and freeze quickly enough – too small and the droplets will simply float away and evaporate. To create the right sized droplets, water must be pressurized, and the correct nozzles used.
Water pressure has the single largest effect on droplet size. It has an inverse relationship with droplet size; the higher the water pressure, the smaller the droplets produced. Most snow guns operate with water pressures in the 100 – 1000 psi range. Commercial snow guns at ski resorts typically operate on the lower end of this range, while home snow guns are often on the higher side.
To produce an even spray pattern with uniform droplet size, the right spray nozzles must be used. While nozzles with smaller orifices and wider spray angles do produce smaller droplet sizes than nozzles with large orifices, the water pressure has been demonstrated to have a greater effect on droplet size. However, the size of the nozzles determines the flow rate through the nozzle and the pressure of the system, so using the correct nozzles is one of the most important things to consider when designing a snow gun.

Temperature
In order for the droplets to cool to below the freezing point, the right atmospheric conditions are necessary. The wet bulb temperature is a measure used by snowmakers to assess snowmaking conditions. The wet bulb is the lowest temperature that can be obtained by evaporating water into the air, until the relative humidity reaches 100% (saturation). Think of getting out of the pool on a sunny day – as the water evaporates from your skin, you feel cold! This is because the evaporation process requires energy (in the form of heat), and that energy is taken from your body.
In the case of the water droplets for snowmaking, the increased surface area also means increased evaporation, but this is a good thing! The evaporation process produces a “microclimate” in and around the droplets (the “plume” of water) with a lower temperature (the wet bulb), resulting in greater cooling of the droplets. Therefore, the temperature of the water droplets should quickly approach the wet bulb temperature.
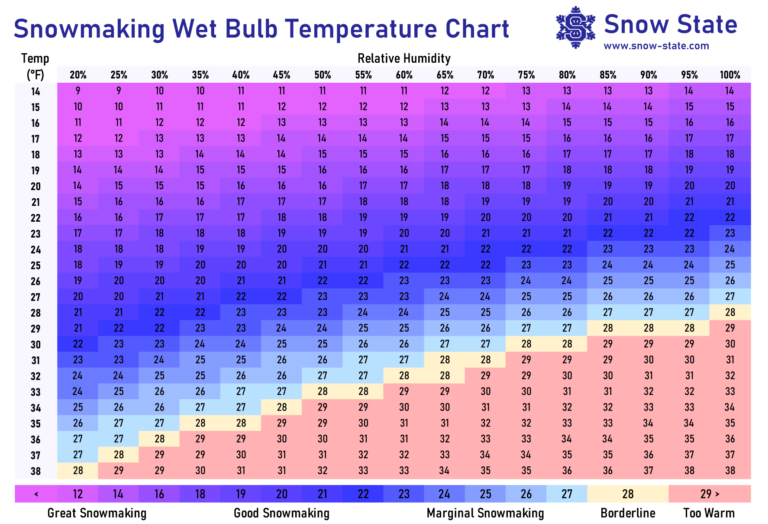
The wet bulb is determined by temperature and relative humidity, and can be approximated with a wet bulb temperature chart. For snowmaking to begin, the wet bulb needs to be around 27°F. Snow can me made at wet bulb temperatures approaching 29°F with properly-designed equipment, but will be very slushy in nature as the snowmaking process is not very efficient at those temperatures, because some droplets won’t have enough time to freeze before reaching the ground.
Most home snow guns will make slushy snow with wet bulb temperatures in the mid-20s, but snow quality will improve to a dry and powdery consistency in the teens and single digits. Ideal conditions for home snowmaking are wet bulb temperatures of less than ~20°F, with little wind.
Nucleation
As mentioned previously, the water droplets will not necessary freeze by themselves, even at low wet bulb temperatures. The droplets need a nucleation site – a place for ice crystal formation to begin. This is because there is a substantial energy barrier required for the droplet to freeze; the water molecules must break hydrogen bonds in order to re-arrange into a lattice structure.
Snowmaking water – whether drawn from a pond or from your home’s water supply – contains many impurities, such as various minerals, dust, and silt. Each of these different compounds has a “nucleation temperature”, which is the temperature at which the compound will nucleate the water droplet and begin the freezing process. Unfortunately, these nucleation temperatures are quite low, so the water droplets will not self-nucleate until conditions are very cold. As a result, another method of nucleation is necessary.
Intuitively, the best nuclei for forming ice is ice itself! To effectively nucleate the water droplets, many small ice crystals must be produced. The nucleation temperature decreases even more for very small droplets, as the probability of finding a nucleating compound in a single water droplet decreases with size. As temperatures approach -40°F (-40°C), the structure of the molecules inside the water droplet becomes more ice-like.
At these temperatures, the energy barrier for freezing can be overcome, and the water droplet can self-nucleate without the aid of any nucleation sites. This process is called homogeneous nucleation. Almost all snow gun designs rely on homogeneous nucleation to effectively “seed” the larger water droplets.
How can temperatures below -40°F be achieved when the ambient temperature is much higher? To accomplish this, compressed air is used. From physics, we know that an expanding gas cools. In the case of snowmaking, large air compressors pressurize air, which results in a temperature increase of the gas. The air is then allowed to cool to near the ambient temperature in the air compressor tank (or with the aid of a heat exchanger on a larger scale) before reaching the snow gun. When the compressed air exits the snow gun, it rapidly expands and cools.
If the compressed air is at a sufficiently high starting pressure, the expansion to atmospheric pressure will result in the air temperature dropping low enough to allow homogeneous nucleation to occur, when a small amount of water is mixed with the air. The required air pressure is usually between 70-120 psi, depending on the temperature of the air and the surrounding atmosphere. A quick calculation shows that 32°F air expanding from 70psi to atmospheric pressure will drop to an astounding -160°F! This is a bit unrealistic for a number of reasons – mixing with the environment and latent heat release from freezing – but nonetheless shows that homogeneous nucleation can occur.
In most snow gun designs, compressed air mixes with a small amount of water to form the ice nuclei via homogeneous nucleation. These ice nuclei then mix with the larger water droplets. When the water droplets make contact with an ice nuclei, they can freeze. This is called heterogeneous nucleation, meaning the freezing of the droplet took place because of the ice nucleus.
Snow Formation
The rate and efficiency of the heterogeneous nucleation process is related to the wet bulb temperature and droplet size. At relatively high wet bulb temperatures, the time for a droplet to freeze is longer. This is why the snow quality is often slushy in marginal snowmaking conditions – not all of the droplets are completely frozen before reaching the ground.
As a result, it is important for a snow gun to be designed so that the water droplets have adequate time to freeze before reaching the ground (hang time). Commercial snow guns are often mounted on tall towers, or use fans to propel the water longer distances in order to maximize hang time and ensure good snow quality.
Additionally, the freezing process releases energy, warming the surrounding environment (latent heat). This becomes an issue when there is a large volume of water in the plume, so the droplets will need more hang time in order to freeze. As a result, the efficiency of the snowmaking process is improved if more snow guns are used (with less flow to each one). This is another advantage of fan guns, as the fan introduces more ambient air into the plume in addition to increased hang time.
Summary
Due the complex physics of ice nucleation, making snow is not as simple as spraying water into the air. High pressure air and water are the essential ingredients to produce the correct droplet size, and ensure proper nucleation. On the home scale, we use pressure washers and air compressors to supply the necessary ingredients.
For the snowmaking process to occur, the wet bulb temperature, which is calculated from temperature and relative humidity, must be at or below 27°F. The efficiency of the freezing process improves at lower wet bulb temperatures, and the snow quality improves as a result. Ready to start making snow? Check out our products and our free plans!